Controlling sulfidity in a modern kraft pulp mill, case study
May 8, 2018
Many years ago, the kraft pulping liquor circuit used sodium sulfate salt as the main make-up source of cooking chemicals. The make-up chemical has given its name to the process in many languages. Back then, the sulfidity reached an equilibrium level that was dependent on the leakages.
Sulfur emissions impact the environment negatively. Reduced sulfur causes smell in the mill’s surroundings and oxidized sulfur contributes to the large-scale acidification of soil, which negatively affects vegetation and biodiversity. Today, both environmental and economic forces have reduced the leakages and most modern mills have practically eliminated sulfur emissions to the atmosphere. In addition, improved pulp washing has reduced the loss of chemicals with the pulp leaving the washers. Reduction of sodium loss from the process is more difficult to achieve due to the fact that sodium is adsorbed on the fiber that leaves the closed liquor cycle. The result of this development is, for most mills, that the equilibrium sulfidity level is higher.
The methods used to control the chemical balance today include e.g. dumping of recovery boiler ash to reduce sulfur content, white liquor oxidation to the oxygen delignification to reduce sodium intake and dumping of chlorine dioxide brine. Although these methods are applied, some modern mills still have difficulties controlling sulfidity.
One development trend in kraft pulping is to create valuable side streams from the process. Such streams are in many cases created by primary acidification with sulfuric acid. Examples are tall oil production and lignin extraction. Introduction of processes like these further disturbs the sodium sulfur balance and the existing means of controlling it becomes insufficient.
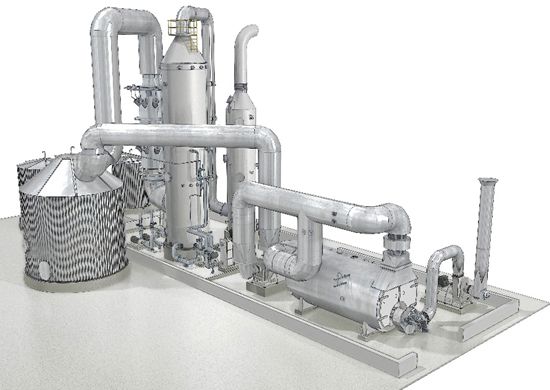
The sulfuric acid plant at the Metsä Fibre Äänekoski mill. The burner and boiler are located on the right, while the catalytic reaction vessel is located to the left in the image. The concentration tower and bisulfite scrubber are located to the right of the reaction vessel. The burner, boiler and the two towers are located inside a building (the lower part of the walls is seen in the image).
Valmet has studied how to expand the sulfidity control toolbox. Our conclusion is that internal sulfuric acid production is the most efficient way to separate sodium and sulfur streams. Therefore, a process which oxidizes sulfur containing concentrated non-condensable gas (CNCG) to relatively concentrated sulfuric acid was developed. The first full-scale plant based on oxidation of CNCG started up late 2017 at Metsä Fibre Äänekoski. The intention is to avoid addition of external sulfur and replace it with internally produced acid. A white paper located HERE describes the plant and scenarios for control of the chemical balance.
For more information on controlling sulfidity in your kraft pulping system, contact your Valmet representative.